A Biosensor is a device that uses a Biological Molecule, such as an Enzyme to detect a Chemical.
The biological molecule produces a Signal (i.e. a chemical signal) which is Converted into an Electrical Signal by a Transducer.
The electrical signal is then processed and can be used to work out other information.
Example:
A Glucose Biosensor is used to determine the Concentration of Glucose in a Solution.
It does this using the enzyme 'Glucose Oxidase' and Electrodes.
The enzyme Catalyses the Oxidation of glucose at the Electrodes. this creates a Charge, which is Converted into an Electrical Signal by the Electrodes (Transducer)
The Electrical Signal is then Processed to work out the Initial Glucose Concentration
Chromatography
The main use is Separating.
Once a solution is separated, we can Identify the Components.
It can be used to Identify Biological Molecules such as Amino Acids, Carbohydrates, Vitamins and Nucleic Acids.
There are loads of different types of chromatography, but we only need to know two, Paper Chromatography and Thin-Layer Chromatography.
Both methods of chromatography have two basic phases:
A MOBILE PHASE
- Where the Molecules Can Move
- in both paper and thin-layer chromatography, the Mobile Phase is a Liquid Solvent, such as Ethanol or Water.
A STATIONARY PHASE
- Where the Molecules Can't Move
- In Paper chromatography, the stationary phase is a piece of Paper.
- In Thin-Layer Chromatography, the stationary phase is a Thin (<0.5mm) Layer of a Solid, i.e. Silica Gel, Glass or Plastic.
They both use the same basic method:
- The Mobile Phase moves Through or Over the Stationary Phase,
- The Components in the mixture spend Different amounts of Time in the Mobile phase And the Stationary phase.
- The Components that spend Longer in the Mobile phase travel Faster or Further.
- The time spent in the different phases is what separates out the components of the mixture.
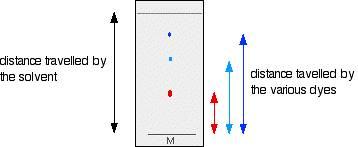
PAPER CHROMATOGRAPHY
- Draw a pencil line near the bottom of the chromatography paper and put a concentrated spot of the mixture of amino acids on it.
- Add a small amount of prepared Solvent in a beaker and Dip the bottom of the paper into it. Cover it with a lid to stop the solvent evaporating.
- As the solvent spreads up the paper, the different amino acids move with it, but at different rates, so they separate out.
- When the solvent's nearly reached the top, Take the paper Out and Mark the Solvent Front (the highest point the solvent has reached), now leave the paper to Dry before analysing it.
- Since amino acids are colourless, Spray them with Ninhydrin solution to turn the amino acids purple, then use the Rf values to Identify the separated molecules.

Spectrophotometer cuvettes I admire this article for the well-researched content and excellent wording. I got so involved in this material that I couldn’t stop reading. I am impressed with your work and skill. Thank you so much.
ReplyDelete